Investigating
Arthropod Distribution and Abundance
as a Basis for
Predicting Foraging Patterns
of the lizard Cnemidophorus tigris
in the
Nicole Addington, Renee Dufek, and Jesse Johnson
BIOL 417a & 417b
Summer Session 2003
Western Washington University
Introduction
Deserts provide a rich opportunity to
study the basic ecological questions about distribution and abundance of
organisms. Desert habitats are
relatively simple systems wherein much of the biota can be observed and
measured readily. The Great Basin Desert,
located principally in Nevada and Utah, is especially tractable because
habitats (of shrubs and bunchgrasses) are more stable and weather is more
predictable (e.g., fewer sheet floods from summer rains) there than in
southwestern U.S. deserts.
Reptiles and arthropods are the prevalent
diurnal animal denizens of most desert habitats. Desert
lizards mostly eat arthropods. Arthropods
are the abundant primary and secondary consumers near the base of the desert
food web. Spatial variation in the desert habitat, as represented by variation
in soil type, ground topography, type and physiognomy of dominant perennial
plants may cause significant variation in arthropod species abundance and
diversity over small spatial scales, such as in mesohabitats and
microhabitats. Gaining knowledge of the spatial
patterns of distribution, abundance, and diversity of the arthropods is
essential to understanding the spatiotemporal patterns of foraging desert
lizards. Knowing the spatiotemporal
patterns of predator and prey is essential to understanding the population consequences
of those interactions, one of the primary goals in ecology.
The western whiptail lizard Cnemidophorus tigris,
is a species whose populations inhabit most deserts in the western
Pit-trapping for
arthropods and standardized searches for the lizard, along with some focal
observations of the lizard documented mesohabitat and microhabitat use by prey
and predator. These spatiotemporal patterns of lizards and
the data from the pit trap study provide preliminary answers to basic questions
and enable several hypotheses to be tested.
The following hypotheses can be
tested propitiously:
·
Hypothesis
1: Larger plants have more standing crop biomass and a greater variety of
nanohabitats than do smaller plants, hence larger
plants will have higher arthropod abundance and diversity.
·
Hypothesis
2: The succulent evergreen perennial Sarcobatus
vermiculatus has more edible biomass for a longer time during the activity
season than the drought-deciduous perennial Artemisia
tridentata. Thus S. vermiculatus will have higher
arthropod abundance and diversity than will an A. tridentata of the same size.
·
Hypothesis
3: The number of arthropods captured in
a pit trap will vary inversely with the distance between perennial plants and
the pit trap.
·
Hypothesis
4: Mesohabitats with more and larger perennial plants will have a greater abundance
and diversity of arthropods captured in pit traps than will mesohabitats with
fewer and smaller perennials. Hence, in
the
·
Hypothesis
5: The lizard Cnemidophorus tigris will selectively forage in mesohabitats and under
plants where their preferred arthropod prey are most abundant.
Study Area and Methods
The chosen study area, the annual study area
for Biol 417a,b, is in the north end of the
The pit traps were placed out in the
open in each of the mesohabitats and under dominant plant species to
investigate spatial variation in arthropod diversity and abundance, and thus, yielding
knowledge to predict the prevalent locations for Cnemidophorus tigris foraging. Pit trapping (methods as in 2002 pit
trapping study for Biol 417a) was done in the three primary mesohabitats
(dunes, sandy flats, and hardpan), and under the two dominant perennial plant
species, Artemisia tridentata (ARTR)
and Sarcobatus vermiculatus (SAVE).
Plants chosen for pit trapping were
quasi-randomly, and a plant was used if it met one of the predetermined size
classes. Microhabitats sampled were small, medium, and
large Artemisia tridentata in the
sandy flats (1 pit trap per small, 2 per medium, and 4 per large), medium Sarcobatus vermiculatus on sandy flats
and dunes, medium A. tridentata on
dunes, and open areas in sandy flats and hardpan. Traps were left out for six
days to collect a representative sample of arthropods. Contents of the pit traps were analyzed in
the laboratory. All arthropods were
extracted from sand and litter, then each arthropod
was identified to the taxonomic level of order, then placed in alcohol-filled
storage vials.
During the field course, students
encountered C. tigris
frequently, thus permitting statistically analyzable data on the mesohabitats
and microhabitats of the lizards when they were first seen. Moreover, some lizards were chosen for focal
observations, thereby providing more detailed information on microhabitat and
nanohabitat use of focal lizards.
Standard plot searches for Cnemidophorus tigris
were conducted on the 150m x 150m study plot daily. When a lizard was first seen, its location
parameters (plot coordinates, substratum, microhabitat)
and behavior were recorded. If the
lizard could not be identified by unique paint marks for each individual
identification lizard, then it was captured, examined for sex and reproductive
condition, weighed, measured, given a unique toe-clip number if it did not
already have one, painted, then released where captured. Initial and repeat sightings of individual
lizards provide information on patterns of habitat use.
Focal observations (20-30 minutes)
were performed on 15 Cnemidophorus tigris as they foraged. Searchers described such
behaviors as shrub entries and exits, changes in direction of travel, changes
in substratum traveled on, details of foraging behavior (e.g., scratching,
digging, climbing), when light and shade patterns striking the lizard changed,
and more. The microclimate and nanoclimate
conditions at the beginning and end of focal observations were recorded.
Results
Out of 278 sightings of C. tigris during
standardized searches of the 150m x 150m plot (June 27-
We analyzed
306 pit-traps for distribution and relative abundance of each major arthropod
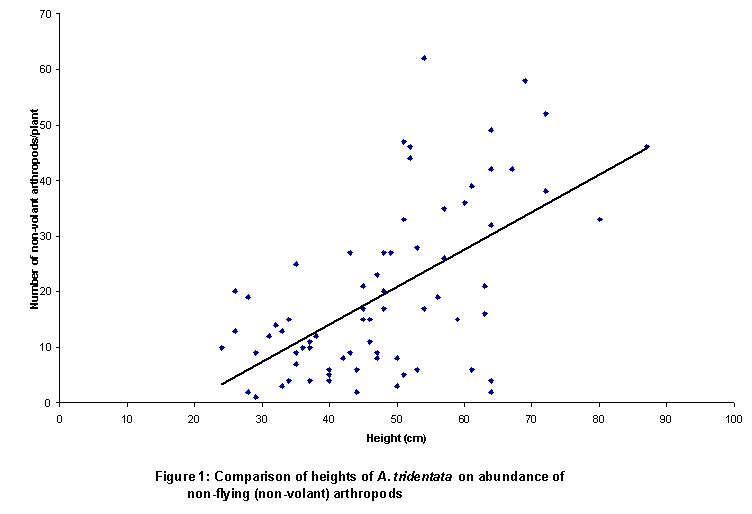
order. More arthropods were found in the
pit traps under taller, larger plants (Figure 1). Ants (taxonomic family Formicidae, in the
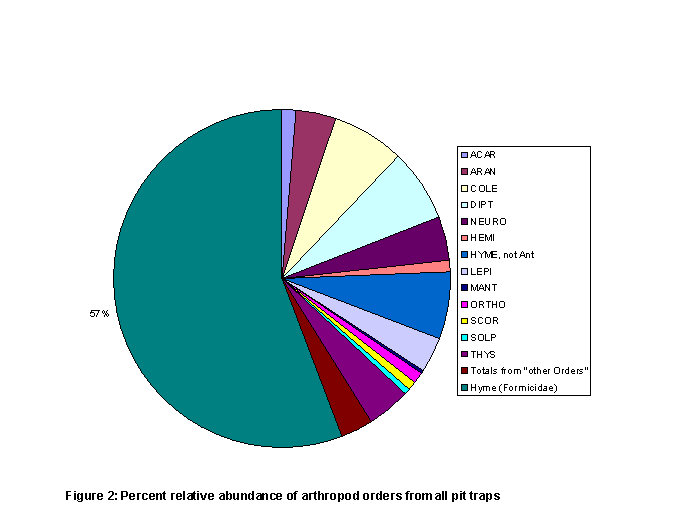
order Hymenoptera) were excluded from the majority of analyses both because
ants are so numerically preponderant in the pit traps and because ants are not
typically a prey item of C. tigris. Ants
accounted for 56% of the total captured insects (Figure 2 and Table 2). Following ants the most abundant arthropods
are in the orders Coleoptera, Diptera, Hymenoptera (non-ants), Thysanura,
Neuroptera, Aranae, and Lepidoptera
(most to least, respectively) collectively comprising 35% of the total
arthropods (Figure 2).
An ANCOVA statistical analysis (P =
0.05) on a per pit trap basis was used to determine if there were significant
differences between the interactions of plant type, size, and mesohabitat. Comparing the total number of arthropods
without Formicidae showed significant differences between the plant types (P =
0.002) and the mesohabitats (p = 0.0001).
These differences between plant types were attributable to differences
among plant categories for Coleoptera, Lepidoptera and non-ant Hymenoptera ( P = 0.001, 0.47 and 0.002, respectively). There were no significant differences
comparing the number of Diptera.
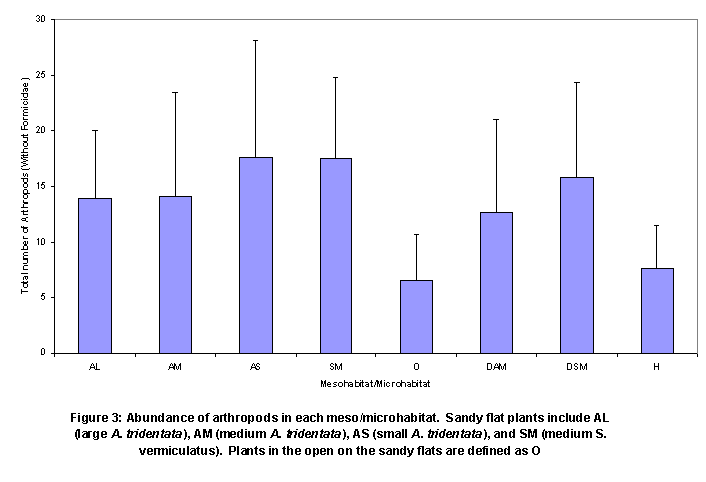
Mesohabitat effects on numbers of
arthropods in pit traps under plants were not obvious (Figure 3). There were no significant differences between
the medium SAVE on the sandy flat
versus the dune. Neither were there
mesohabitat differences in arthropod abundance for the medium ARTR. The arthropod numbers were similar for open
pit traps in sandy flat and hardpan.
Small, medium,
and large ARTR had relatively similar numbers of arthropods per pit trap (no
significant differences) and all plants had significantly more arthropods per
trap than did lone pit traps in the open (about 1 m from nearest plant). When volants were included, medium Sarcobatus vermiculatus (SAVE) had
nearly a significantly greater number of arthropods (Figure 3 and Figure
4).
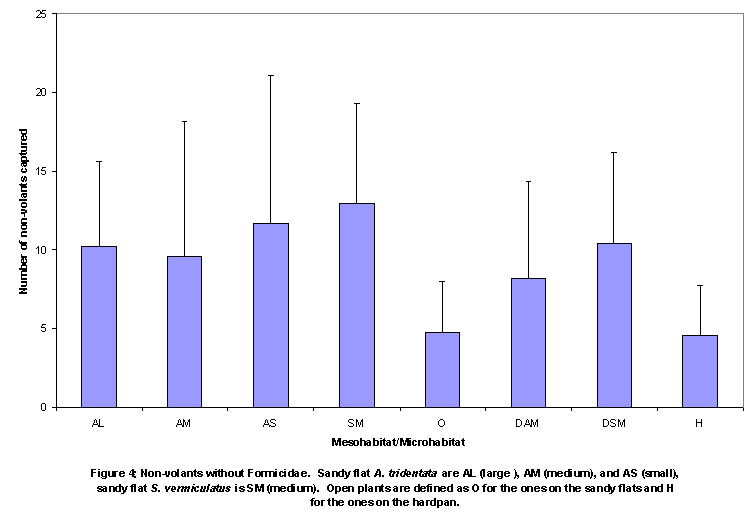
Statistically significant differences
in numbers of arthropods trapped per plant existed between all combinations of
sandy flat microhabitats: Within ARTR,
as plant size increased, the number of pit-trapped arthropods caught under the
plant increased. Areal projection of
plant cover was compared with total numbers of arthropods, without Formicidae,
for all the ARTR size groups on the sandy flats. (Figure 5). Statistically (t-tests, P < 0.05), greater
numbers of arthropods were found in a) pit traps under small plants than in
open pit traps (P = 0.0001), b) under medium size plants than under small
plants (P = 0.018), and c) under large plants than under medium plants (P =
0.00001).
We made the tentative assumption that
the mean number of arthropods in the sandy flat open pit traps, 5, represented
the general habitat effect on numbers of arthropods under the plant. Thus, we subtracted 5 arthropods from the
mean for each of the three size classes of ARTR. The result is that although the larger size
class covered four times the area that a small size class did, the larger size
class had five times more arthropods than did the smaller size class. Hence, there may be an increase in the number
of arthropods per unit area of cover with increasing plant size.
Discussion and
Conclusion
Some
of our predictions were not supported by our data from pitfall traps. For example, we predicted that pit traps
under plants in the dune mesohabitat would capture more non-ant arthropods than
would pit traps under plants of the same size and species in the sandy flats
mesohabitat. Our results showed that pitfall traps under perennial plants of
the same size and species had similar numbers of arthropods, despite the
different habitat. We also predicted
that among plants of similar size, pit traps under the succulent,
heavily-leaved SAVEs would have more arthropods than would pit traps under the
less palatable, sparsely-leaved ARTRs.
Although there was a trend, the difference was not statistically
significant; perhaps we did not sample enough SAVEs.
Comparisons of captures rates of
arthropods in pit traps among plants varying in size produced results ambiguous
results as well. The total number of
arthropods captured in all pit traps under a plant varied directly with plant
size. There were significant differences in number of non-volant, non-ant
arthropods among the small, medium, and large ARTRs on the sandy flats. It is possible, however, that the greater
number of pit traps under the larger plants was partially or entirely the
reason for higher numbers of arthropods captured under larger plants. Thus, our analysis of the number of
non-volant, non-ant arthropods caught per pit trap revealed no significant
differences among plants varying in size.
The only significant differences in
arthropod numbers were between pit traps in the open and pit traps under
plants. Out in the open, there is no
refuge on the ground surface from the extreme heat of
We
infer from the data in our standard plot surveys, observations of western
whiptails, and pit trapping that for the period of our study these lizards
neither did nor should use plant size as a cue for greater food
availability. The ARTRs were the most
common species visited and foraged in by C. tigris. But frequency of foraging under ARTRS may be
the simple result of ARTRs being the most common species of perennial on the
plot.
Although pit traps are one of the
best ways to estimate relative arthropod abundance and density, it is unlikely
that pit traps capture numerically representative samples of all the arthropods
that C. tigris tends to prefer. These lizards eat a lot of arthropods that
are hidden in soil and leaf litter, such as termites, quasi-sedentary larvae,
and profoundly sedentary pupae and eggs (
An experiment varying the number of
pit traps within and among perennial plants varying in sizes may definitively
reveal whether larger perennial plants harbor more arthropods per unit area of
plant cover than do smaller perennials.
Moreover, larger sample sizes should be used to compare arthropod
abundance under different species of perennials.
Our preliminary analysis of a limited
portion of the available C. tigris foraging data is that there is too much variation
in lizard behavior relative to the sample size analyzed. We anticipate that full analyses of field
course foraging data from (1999 and 2003) will reveal some understanding of the
spatiotemporal patterns of foraging in Cnemidophorus
tigris.
Table 1.
Summary data from focal observations on Western Whiptails (N=8).
|
avg ARTR height |
shortest ARTR |
tallest ARTR |
# of plants |
time observed (sec) |
total dist traveled |
avg dist traveled |
est time under ARTR (sec) |
% est time under ARTRs |
|
0.8125 |
0.75 |
1 |
5 |
1410 |
9 |
0.9 |
606 |
0.429787 |
|
0.6071 |
0.25 |
0.75 |
7 |
1153 |
24.25 |
1.68 |
846 |
0.733738 |
|
0.5833 |
0.5 |
0.75 |
5 |
1240 |
11 |
0.5 |
709 |
0.571774 |
|
0.6944 |
0.5 |
1 |
9 |
1302 |
27.25 |
3.41 |
1260 |
0.967742 |
|
0.75 |
0.25 |
1.75 |
19 |
1179 |
47.75 |
2.98 |
522 |
0.442748 |
|
0.3214 |
0.25 |
0.5 |
7 |
1500 |
51.75 |
2.88 |
483 |
0.322 |
|
0.5794 |
0.25 |
1 |
17 |
2259 |
91.75 |
2.62 |
1180 |
0.522355 |
|
0.6964 |
0.25 |
1 |
14 |
1416 |
52.25 |
2.01 |
1122 |
0.792373 |
|
0.6046 |
|
|
10.375 |
|
|
|
841 |
0.597815 |
Table 2.
Arthropods captured in 306 pit traps over six days in the
|
A C A R |
A R A N |
C O L E |
D I P T |
N E U R |
H E M I |
H Y M E not Ant |
L E P I |
M A N T |
O R T H |
S C
O R |
S O L P |
T H Y S |
Other Orders |
H Y M E Ant |
|
137 |
358 |
675 |
671 |
376 |
129 |
596 |
313 |
27 |
120 |
77 |
47 |
400 |
307 |
5324 |