Spatiotemporal patterns of desert grasshoppers
and their predator, the leopard lizard, Gambelia
wislizenii
Janet Cray, Colleen Duck, and Colin
Hume
Biol 417a,b, Summer Session 2002
(poster
edited by Dr. Anderson to fit website format)
Introduction
Across a south-to-north
gradient in the deserts of the western U.S.A, body sizes decrease, population
densities increase, and diets shift from mostly lizards to mostly grasshoppers
in populations of the long-nosed leopard lizard Gambelia wislizenii. Study
of a population of G. wislizenii near
the northern and upper elevation extremes of the species’ geographic range
should provide a useful base of knowledge to compare with data on southern
populations, and thus lead to an understanding of the causes for the geographic
trends.
A convenient study locale is
in the northern
Arthropods appear to be abundant
in the study area, and grasshoppers appear especially easy to encounter. Spatiotemporal patterns of the distribution
and abundance of grasshoppers in the study area may influence the
spatiotemporal patterns of Gambelia. We decided to investigate the spatiotemporal
distribution of grasshoppers in the study area and compare sighting frequency
of Gambelia with the spatiotemporal
patterns of grasshoppers. We also decided to observe grasshopper escape
behavior during our encounters with an anticipation that grasshopper
evasiveness may vary with ontogeny, time of day, and plant structure, as well
as among grasshopper species. The
spatiotemporal distributions, abundances, and evasiveness of grasshoppers are
expected to influence the food acquisition behavior of Gambelia wislizenii.
Methods
- After a few days of anecdotal
surveys we decided to compare among four obviously different habitats for
their spatiotemporal patterns of grasshoppers and Gambelia wislizenii: 1) sage-dominated habitats upslope on
compact sand and gravel (upper transition), 2) sage and greasewood as
co-dominants downslope on the looser sand of sandy flats, small dunes,
albeit mixed with some hardpan depressions (lower transition), 3) larger
dunes further downslope, and 4) grassy hardpan salt flats in the valley
basin. We used areas of
approximately 40m x 400 m in the grassy salt flats and the upper and lower
transitional. Out of 11 dunes available,
3 dunes were randomly selected, and a 150 m long section of each of the 3
dunes was randomly chosen for the study.
Plant species designations: Table 1.
- Distribution, abundance,
areal cover and volume of each perennial plant species was estimated with three
50m line intercepts on each plot (and on dunes verified by measuring all
perennials on six 5m2 quadrats on each of three dunes); lines
and quadrats were randomly chosen on each habitat area.
- Each grasshopper and Gambelia sampling episode covered a
unique 10 x 50 area and lasted no longer than an hour. Time taken to cover 10 x 50 m areas in
all four habitats in a collection period depended on number of grasshoppers
encountered, but generally required about 4 hours. Collection time periods were 0730 to
1200 and 1530 to 2000. We decided
on 9 collection episodes per habitat per collection time period. At beginning and end of each collection
episode at each site, these microclimate data were collected: wind speed,
ground surface temperature, air temperature at 2m above ground. Chance encounters with Gambelia were noted.
- Grasshopper collection
methods: 3 persons with sweep nets
spread out side by side to cover a “foraging width” of 10 m. The general method was to look for a
grasshopper, and if none were readily apparent, then to brush the bushes
with the net handles or sweep the open ground with the nets to flush them
out. Grasshoppers spotted on the
bushes were caught using the larger (11 cm) snap-lock vials, quickly
trapping them into the vial with the lids.
Grasshoppers spotted on the ground were captured by quickly
slamming the nets down over them, which often caused them to jump up into
the nets for easier collection. If
the grasshoppers were flushed from a perennial, a flagging weighted with a
large washer was tossed on the ground near the initial sighting location
of the grasshopper. Retractable metal
measuring tapes were used to measure the horizontal and vertical distances
of the first hop or flight.
- Data taken for each
grasshopper: date, plot, time,
species, adult/nymph, initial behavior, head direction, escape direction,
escape angle (oblique or straight, towards or away), horizontal and
vertical hop distances, capture vial number, initial substrate and final
substrate. An effort was made to
note and identify any grasshoppers that escaped. All grasshoppers collected were placed
in vials and preserved in a mixture of ethylene glycol and isopropyl
alcohol.
Results I.
·
Dunes:
Quadrat technique on the dunes show the dominant species to be the
greasewood Sarcobatus vermiculatus
(SAVE) for both percent cover and percent volume, (Figs. 1, 2). The line
intercept technique also revealed SAVE as the dominant species.
·
Upper Transition: The line intercepts technique shows the
dominant species to be the Basin Big Sagebrush Artemisia tridentata (ARTR, Fig. 1).
·
Lower Transition: The line intercept
technique shows the dominant species to be Basin Big Sagebrush Artemisia tridentata (Fig. 1).
·
Grassy Salt Flats: This area had such low perennial plant
diversity and percent cover that a rough estimate of 5% Sarcobatus vermiculatus (Greasewood) and 5% Panicum occidentale (PAOC) was used.
Results II. Microhabitats
compared for grasshopper abundance
·
Out of 701 total grasshoppers observed, the grassy
flats harbored significantly
fewer grasshoppers (Fig 3).
·
Out of all grasshoppers observed, 51% were first seen
on plants, 43% were first seen on the ground in the open and for 4% the
location of first sighting was uncertain (Fig. 4).
·
The nymphs were found more on the plants, but adults
(presumably involved in courtship) were found more on the ground in the open
(Fig. 5).
·
Of 289 grasshoppers found on plants, most were on Artemisia tridentata and Sarcobatus vermiculatus (Fig. 6).
·
Grasshopper
species richness was highest on PAOC, SAVE, and ARTR (Fig 7). Note that the grass Panicum occidentale (PAOC), was the prevalent plant on the salt
flats, and that one portion of the salt flats habitat was very close to a dune;
hence, the wind and proximity of dunes may have inflated the species list of
“resident” grasshoppers with transients.
SAVE was dominant on dunes and ARTR was dominant on the other two
habitats.
·
Although there was no correlation between
the volume of a plant and the number of grasshoppers on the plant (perhaps a
sample size or methods problem), there was a significant relationship between
percent cover of a perennial plant species on a plot and the number of
grasshoppers found on that plant species on the plot (R2 = 0.545
with a p value of 0.044).
·
Fifteen tentative taxa of grasshoppers were
designated (Table 2, includes nymph, adult male, and adult female categories)
and only about one-third could be considered “common” (Fig. 8)
·
Among initial evasion behaviors of all grasshoppers,
the most common was hopping (Fig 9) although for species T5, T6, T7, T9 flying
was the prevalent predator evasion behavior. Note that sample sizes were very small for
some grasshopper species.
·
Evasion directions and distances of
grasshoppers revealed horizontal movements to be several times greater than
vertical movements (Fig 10); sample sizes were similar with about 8 individuals
sampled per movement type per species.
Results
III. Microhabitats compared for Gambelia
wislizenii abundance.
·
Out of the limited number of G. wislizenii observed during grasshopper collection periods most
were seen in the lower transitional habitat, but sample sizes were low. Hence we used data on chance encounters with Gambelia on the field course study site,
in the same habitat as LT. Most Gambelia
were first seen in the open rather than directly under plants (Fig. 11). Note also the microhabitats of adult
grasshoppers (Fig 4).
·
Of the G.
wislizenii that were observed under plants, most were under Sarcobatus vermiculatus and Artemisia tridentata (Fig. 12). Note also the grasshopper abundance related
to plant cover (Fig 5).
Discussion and
Conclusions
During our short-term study, adult grasshoppers were
more common on the ground in the open, in easy view for the lizard, but as a
combined total of nymphs and adults there were more grasshoppers on the
perennial plants. Both nymphs and adults
on plants, however, should be less visually obvious than adult grasshoppers on
the ground in the open. Moreover, grasshopper
nymphs were found most often in and near the top of perennial shrubs. The large
size of adult grasshoppers and female adult grasshoppers laden with eggs should
be more preferred than the smaller nymphs by Gambelia wislizenii. Adult
grasshoppers, however, can fly and can evade a pursuing Gambelia more easily than can nymphs,
hence nymphs and the adults on the perennials should not be ignored by Gambelia. Moreover, earlier in the activity season most
grasshoppers would be nymphs, and . Thus, early in the activity season either Gambelia should station themselves near
the plant perimeters to catch grasshopper nymphs or Gambelia should be stationed under plants to facilitate ambushing of
lizards. But during our study adult
grasshoppers were common in the open (Figure 5), hence we expected Gambelia to be stationed more in the
open where the adult grasshoppers were.
Indeed, microhabitat use data from sightings of Gambelia wislizenii on
the field course study site, the same habitat as the LT habitat supported this
prediction (Figure 11).
We caught more grasshoppers on the two dominant perennial
plant species than
on other perennial plant species (Figure 6).
Hence, we expected Gambelia wislizenii to be positioned more closely
to these two dominant perennials, Basin Big Sage (Artemisia tridentata) and Greasewood (Sarcobatus vermiculatus), than near other plants on the field
course study site, in the LT habitat.
Indeed, our expectations were met (Figure 12).
Too few Gambelia were seen among all habitats
during our grasshopper collection episodes to discern confidently a
differential habitat use by Gambelia. But 1) our anecdotal lizard sightings data, 2)
fewer grasshoppers seen per unit area in the salt flats, and 3) very little
shade in the salt flats, all support a prediction of fewer Gambelia in salt flats than in the other three habitats. The prediction is supported by data in J. Steffen’s
master’s thesis; moreover, the apparent higher abundance of Gambelia on the LT also are corroborated Steffen’s data. More detailed observations and analyses,
however, of spatiotemporal patterns of grasshoppers, prey lizard species, and Gambelia among mesohabitats (e.g., dune,
hardpan, sandy flats), microhabitats (e.g., at perennials) and nanohabitats
(e.g., precisely where on or under perennials) on the field course study site
should help elucidate the causes and consequences of Gambelia spatiotemporal patterns, feeding rates, body sizes, and
diets across the geographic range of G.
wislizenii.
Tables and
Figures
Table
1. Plant and Substratum Names and Codes
|
Plant Code |
Species Identification |
Common Name |
|
ACHY |
Achnatherum hymenoides |
Indian rice grass |
|
ARSP |
Artemisia spinescens |
Bud Sage |
|
ARTR |
Artemisia tridentata |
Basin big sagebrush |
|
ATCA |
Atriplex canescens |
Four wing saltbrush |
|
ATCO |
Atriplex confertifolia |
Shadscale |
|
BRTE |
Bromus tectorum |
Cheat Grass |
|
CWD |
|
Coarse woody debris |
|
DISP |
Distichlis spicata |
Salt grass |
|
ERNA |
Ericameria nauseosa |
Gray rabbitbrush |
|
ERVI |
Ericameria viscidifloria |
Green rabbitbrush |
|
GRSP |
Grayia spinosa |
Spiny hopsage |
|
PAOC |
Panicum occidentale |
Witchgrass |
|
SAVE |
Sarcobatus vermiculatus |
Greasewood |
|
TEGL |
Tetradymia glabrata |
Littleleaf horsebrush |
|
TESP |
Tetradynia spinosa |
Cat claw horsebrush |
|
HP |
|
Hardpan substratum |
Table 2: Identification of grasshoppers caught in
|
SPECIES CODE SM1 A1 |
PROBABLE GENUS
Melanoplus |
SUBFAMILY OF ACRIDIDAE Melanoplinae |
|
SM1 A2 |
Melanoplus |
Melanoplinae |
|
S? N1 |
Melanoplus |
Melanoplinae |
|
SM2 A1 |
Melanoplus |
Melanoplinae |
|
SM2 A2 |
Melanoplus |
Melanoplinae |
|
SM2 N1 |
Melanoplus |
Melanoplinae |
|
S? A1 |
|
Melanoplinae |
|
S? A2 |
|
Melanoplinae |
|
P1 A1 |
Paropomala |
Gomphocerinae |
|
P1 A2 |
Paropomala |
Gomphocerinae |
|
P1 N1 |
Paropomala |
Gomphocerinae |
|
P1 N2* |
Paropomala |
Gomphocerinae |
|
P2 A1 |
Paropomala |
Gomphocerinae |
|
P2 A2 |
Paropomala |
Gomphocerinae |
|
P2 N |
Paropomala |
Gomphocerinae |
|
C1 A1 |
Cordillacris |
Gomphocerinae |
|
C1 A2 |
Cordillacris |
Gomphocerinae |
|
C1 N1 |
Cordillacris |
Gomphocerinae |
|
T3 A1 |
Trimerotropis |
Oedipodinae |
|
T3 A2 |
Trimerotropis |
Oedipodinae |
|
T4 A1 |
Trimerotropis |
Oedipodinae |
|
T5 A1 |
Trimerotropis |
Oedipodinae |
|
T5 A2 |
Trimerotropis |
Oedipodinae |
|
T6 A1 |
Trimerotropis |
Oedipodinae |
|
T6 A2 |
Trimerotropis |
Oedipodinae |
|
T7 A1 |
Trimerotropis |
Oedipodinae |
|
T7 A2 |
Trimerotropis |
Oedipodinae |
|
T8 A1 |
Trimerotropis |
Oedipodinae |
|
T9 A1 |
Trimerotropis |
Oedipodinae |
|
T9 A2 |
Trimerotropis |
Oedipodinae |
|
T10 A1 |
Trimerotropis |
Oedipodinae |
|
CO1 A1 |
Conozoa |
Oedipodinae |
|
CO1 A2 |
Conozoa |
Oedipodinae |
|
ST? A |
|
Melanoplinae |
|
ST? N |
|
Melanoplinae |
|
G? A |
|
Gomphocerinae |
|
G? N |
|
Gomphocerinae |
|
P? A |
|
Gomphocerinae,paropomal |
|
P? N |
|
Gomphocerinae,paropomal |
|
T? A |
|
Oedipodinae |
|
T? N |
|
Oedipodinae |
|
TET A |
|
Tettigionidae |
|
TET N |
|
Tettigionidae |
Life stage, sex, and species designations assigned by
Colin Hume, with the aid of the following references:
1) Book: North American Grasshoppers by Daniel Otte,
2)
Website: Grasshoppers of
3) Research Report: OSU survey species list
for the
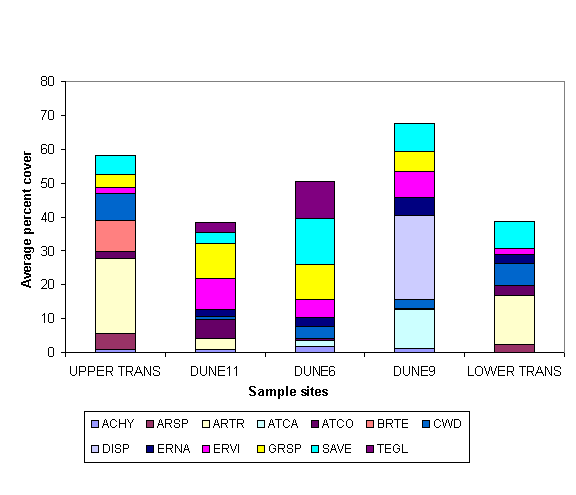
Figure 1. Comparisons
among perennial plant species for percent
cover, on three of four habitats studied; dunes
are
shown individually; see
Table 1 for plant species codes.
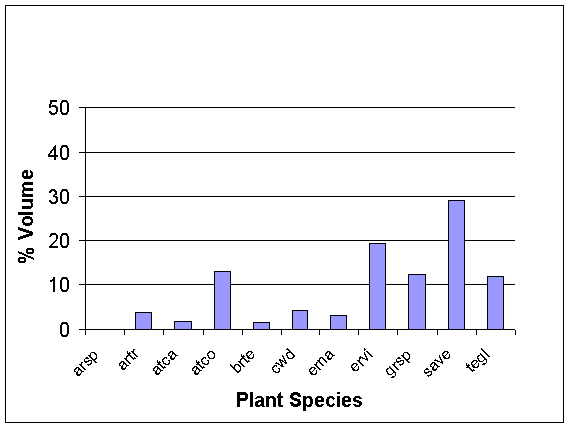
Figure
2. The combined averages for measures of relative %
volume
of each
perennial plant species among all perennials on three
dunes, as
measured by six 5m2 quadrats on each dune.

Figure 3. The mean number of grasshoppers observed per similar
area
traversed during
collection episodes on each of four habitats sampled
in the
LT=lower transition, UT = upper
transition (LT is the main field
course study
area, it is an ecotone between sage-dominated vegetation
upslope to greasewood-dominated
vegetation downslope). The mean
for SF is
significantly less than the other sites, which do not differ
from each other
(ANOVA, F ratio = 8, error df = 20. Trt
ms = 5,
N = 701 grasshopper sightings).

Figure 4. Grasshopper microhabitat use; combined
observations
of all grasshoppers
from all four habitats.

Figure 5. Microhabitat use by nymphal and adult
grasshoppers;
combined observations from all four habitats.

Figure 6. Relative abundance of grasshoppers among
perennial plant species;
these are combined observations combined from
all four habitats.

Figure 7. Grasshopper species richness on perennial plants
and substrata;
see Table 1 for
designations.
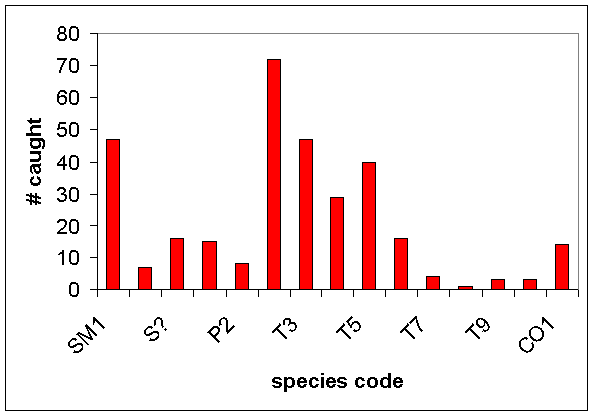
Figure 8. The total number of each grasshopper
“species” caught among
four habitats
in the
See Table 2 for grasshopper species
codes.

Figure 9. Distribution
of evasion behaviors of grasshoppers,
N = 11.4 + 12.2 evasions per species, range 1-41;
see Table 2 for grasshopper species codes.

Figure 10.
Horizontal and vertical evasion distances by grasshoppers.
Sample
sizes per species are 8 + 8.8 (range 0-32) horizontal
evasions and 7.6
+ 10.3 (range 1-31) for vertical evasions.
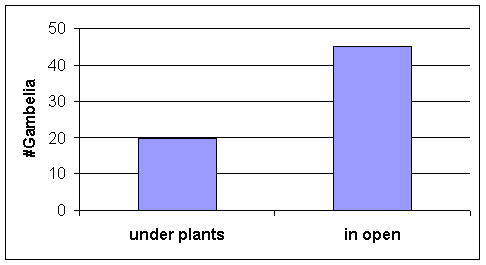
Figure 11. Gambelia
microhabitat use: location of each lizard
when it was first seen during chance encounters
on
the field
course study site, in the LT habitat;
N = 65 lizards encountered.
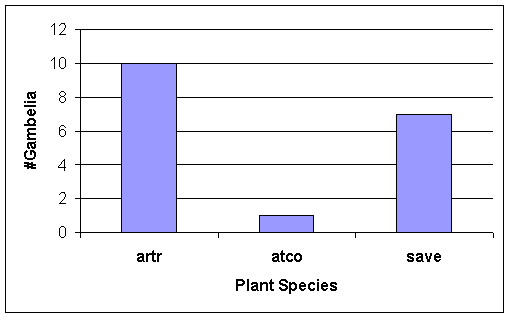
Figure 12. Use of plant microhabitats (= on ground under plant)
by Gambelia
wislizenii. N = 18 observations of
G. wislizenii under a
perennial plant when the lizard
was first seen during a chance encounter.
See Table 1 for plant codes.